Abstract
Background Since the introduction of tyrosine kinase inhibitors (TKIs), the life expectancy of patients with chronic myeloid leukemia (CML) approaches that of the age-matched population. According to the Surveillance, Epidemiology, and End Results (SEER) Program of the National Cancer Institute, about 30% of CML patients are 75 years old and above, but only 3.8% of this age group is represented in clinical trials.
In this study we characterize and asses the outcome of the elderly patients diagnosed with CML at the age of ≥ 75 years.
Methods We conducted a retrospective study, using electronic medical records of consecutive patients diagnosed with CML at the age of ≥ 75 years between January 1st 2002 and December 31st 2021 at the Rabin and the Sourasky Medical Centers in Israel.
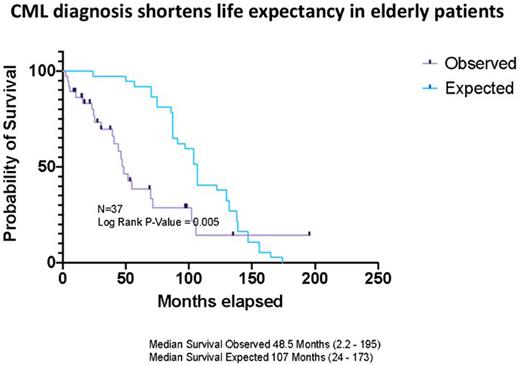
The 1 and the 5-year overall survival (OS) for the whole cohort and for the octogenarians, namely those above 80 years, were calculated. To test whether the diagnosis of CML shortens life expectancy, we estimated the median OS of the whole cohort at the time of CML diagnosis, hereafter termed expected OS, using the life expectancy according to the central bureau of statistics (CBS). The expected and the observed median OS were plotted using Kaplan-Meier curves and were compared using Log-rank.
The study was approved by the local Institutional Review Boards.
Results A total of 37 patients ≥ 75 years were diagnosed with CML between 2002 and 2021. The median age was 81 (range: 75 - 100) years, and 22 patients were octogenarians. Comorbidities at the time of CML diagnosis were documented in 36 patients (97%), with a median number of 3 (range: 0-6) comorbidities including cardiovascular risk factors in 35 patients and cardiovascular/cerebrovascular diseases in 18 patients. Most patients (n=35) were diagnosed in chronic phase (CP) CML, while only 2 patients were diagnosed in accelerated phase CML. EUTOS-LTS (ELTS) risk score was calculated in 29 patients, mostly high (41%) and intermediate risk (55%).
All patients received imatinib as first line of treatment with a median dose of 400 (range: 100-600) mg and the median time on imatinib was 9 (range:1-137) months. While 22 patients (60%) discontinued imatinib due to either intolerance (n=17) or resistance (n=5), including 1 patient who progressed to blast crises (BC), 12 patients required dose reduction. Most patients (n=16) initiated second-line treatment with 2nd generation TKI (dasatinib-12, nilotinib -3 and bosutinib-1), while 5 received hydroxyurea as palliative care and 1 patient was lost to follow-up. Only 4 patients reached a third line of treatment (bosutinib-2, nilotinib-1, ponatinib-1) due to intolerance - 3 patients and resistance - 1 patient.
In 28 patients (85%) response was assessed by PCR. The median time to maximal response was 16 (range:4-98) months. Of 28 patients, 85% (n = 24) achieved complete cytogenetic response (CCyR), 75% (n = 21) achieved major molecular response (MMR) and 54% (n = 15) achieved deep molecular response (DMR). Treatment free remission was documented only in 1 patient with molecular remission at 4 years after nilotinib discontinuation.
The median follow-up duration, from diagnosis to last follow-up visit/death, was 38 (2-198) months. During follow-up, 27 patients (73%) reported on adverse effects (AEs) including CV AEs in 4 patient and 22 patients died, including 4 (11%) patients
With progression to advanced phase CML. Causes of death were known in 14 patients and the main causes were infection (n=5) and CV complications (n=4).
The 1 and the 5-year OS for the whole cohort was 89% and 54%, respectively and for the octogenarians, 91% and 50%, respectively. The median observed OS was 48.5 (2.2-195) months compared with the median of 107 (24-173) months expected OS.
Furthermore, in a sub-analysis of patients diagnosed with CML after 2009, when multiple TKIs became available in Israel for patients with resistance/intolerance to imatinib, the observed OS was still reduced in comparison to the expected OS.
Conclusions In our study, most patients older than 75 years presented with high/intermediate risk CP CML. All were treated with imatinib as first line of treatment and the majority achieved CCyR and MMR. Nevertheless, our study supports reduced life expectancy in the very elderly patients with CML.
Disclosures
Raanani:BMS: Consultancy; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal